How AI can help understand sarcomere structures in hiPSC-CMs?
August 3, 2024 | By Tam Phuc
A research team at the VinUni-Illinois Smart Health Center (VISHC) has recently developed an innovative AI-based framework called SarcNet to analyze and evaluate sarcomere structure in hiPSC-CMs. This innovative tool will be a stepping stone to solving critical biomedical challenges with practical and effective solutions.
In the quest to find better treatments for heart disease, researchers often rely on their understanding of human cell lines or animal models. However, these methods fall short of accurately mimicking human heart conditions. The new technology using human-induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CMs) to recap the native-like heart cell, offers a better way to model human heart conditions in the lab. While there are many ways to quantify hiPSC-CMs, analyzing their sarcomeres—the basic contractile units of heart muscle cells—is crucial for understanding heart function. However, the quantitative analysis of sarcomere structures in hiPSC-CMs has been challenging due to the complexity, microscopic scale and variability of these cellular components. A recent AI-based framework published by a research group at VinUni-Illinois Smart Health Center (VISHC) brings us closer to overcoming this challenge and paving the way for more advanced tasks.
Human induced pluripotent stem cells (hiPSCs) can be created by reprogramming healthy or patient-derived somatic cells and then differentiated into all subtypes of cardiomyocytes (hiPSC-CMs). hiPSC-CMs have a wide range of applications, which highlighted was proven effective for drug screening, modeling diseases, and advancing personalized medicine. Therefore, it is essential to create an automated and high-throughput framework for measuring the maturation of hiPSC-CM. However, quantitative approaches for tracking hiPSC-CMs development are currently limited by labor-intensive and error-prone traditional methods like manual annotation or Fourier transform analysis.
The sarcomere, the contractile unit of the cardiac myocyte, consists of parallel, cross-striated bundles of thin filament containing actin, tropomyosin, and the troponin complex, along with thick filament primarily composed of myosin and its supporting proteins (Figure 1).
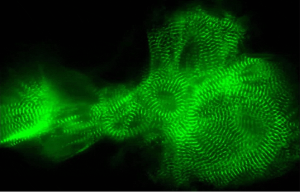
Figure 1: Sarcomere structure (Harvard Medical School).
The organization and structure of sarcomeres directly correlate with the contraction capabilities of adult cardiomyocytes. Mature hiPSC-CMs, which are longer and exhibit a higher degree of structural organization, reflect the advanced functional characteristics necessary for proper cardiac function. Evaluating sarcomere configuration helps assess the functional and developmental state of the hiPSC-CMs, providing insights into their readiness for use in research and potential therapeutic applications.
The team, led by VISHC’s PhD students Huyen Le and Khiet Dang, Tien Lai, and supervisors from the College of Engineering & Computer Science, the College of Health Science, at VinUniversity, has developed an innovative AI-based framework called SarcNet to analyze and evaluate sarcomere structure in hiPSC-CMs (Figure 2). This work has recently introduced in the 10th International Conferences in Vietnam on the Development of Biomedical Engineering and attracted attention from the research community.

Figure 2. Our overall framework used to quantify sarcomere structure organization.
“SarcNet can make a significant impact in several key applications,” Huyen said. “For example, SarcNet facilitates fundamental research in cardiac biology and hiPSC-CMs cultural protocol development by providing detailed quantitative analyses of sarcomere maturation. In drug screening, SarcNet enables rapid and accurate assessment of new drug candidates’ effects on cardiac cells.”
SarcNet showed superior performance compared to other methods. It achieved the unprecedented, highest Spearman correlation of 0.831, indicating a strong association between predicted and ground truth scores for sarcomere organizations. The method also maintained high performance, which speeds up training and inference processes by reducing the number of features while retaining a performance of 0.825.
“The integration of computer vision and feature engineering enables this AI-based framework to overcome the limitations of traditional methods through automated, high-throughput analysis, providing consistent, reliable results while accurately detecting complex sarcomere patterns across diverse samples.”, Huyen added.
The first author of the paper also mentioned the next steps to improve SarcNet’s performance. “Our latest experimental results indicate significant improvements across all performance metrics with the new model. We are in the final stages and look forward to sharing our new findings soon,” she said. In the long term, the team plans to expand the research by building a benchmark dataset and evaluating the effects of a wide range of drugs on hiPSC-CMs. This innovative tool will be a stepping stone in VISHC’s commitment to solving critical biomedical challenges with practical and effective solutions.
Editor’s Note: The full text of the paper can be accessed at 2405.17926 (arxiv.org)